Tap into our team of experts and tools to help you get your organization’s policies and procedures up to date with the latest compliance and regulatory standards.
Try Sierra Policies Now
We've indexed the Code of Federal Regulations (CFRs) that apply to life science companies including medical device manufacturers, pharmaceuticals, and clinical research organizations.
Search our knowledge base of GxP standards and templated policies and procedures.
Our traceability database cross references FDA guidelines, GxP standards, and industry best practices.
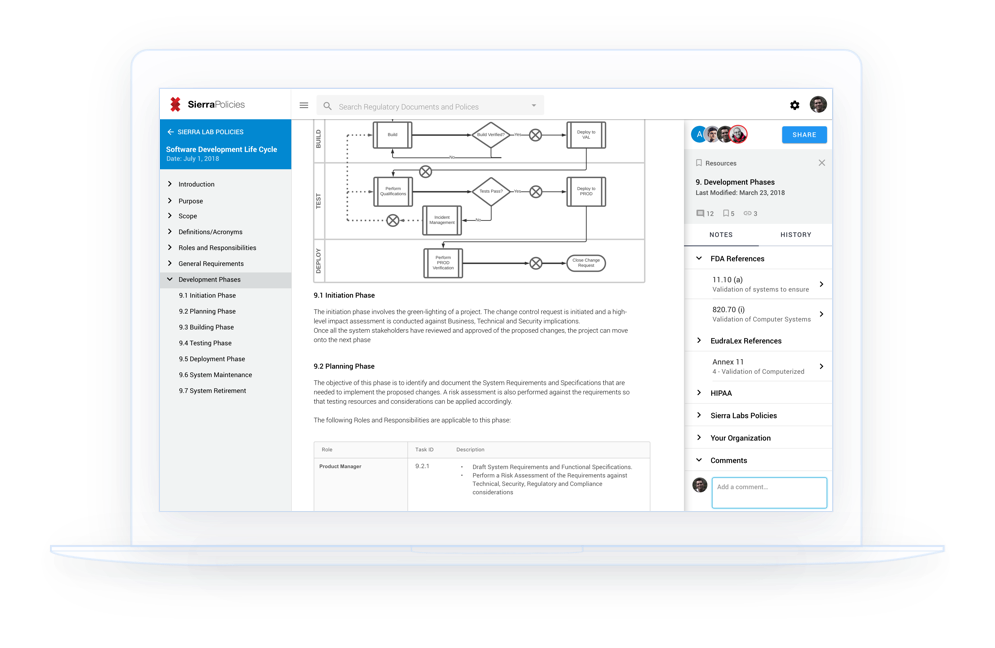
Team pages provide an overview of all the policies created by your team. Collaborators can work together in researching new regulations, creating new policies, or making changes to existing policies.
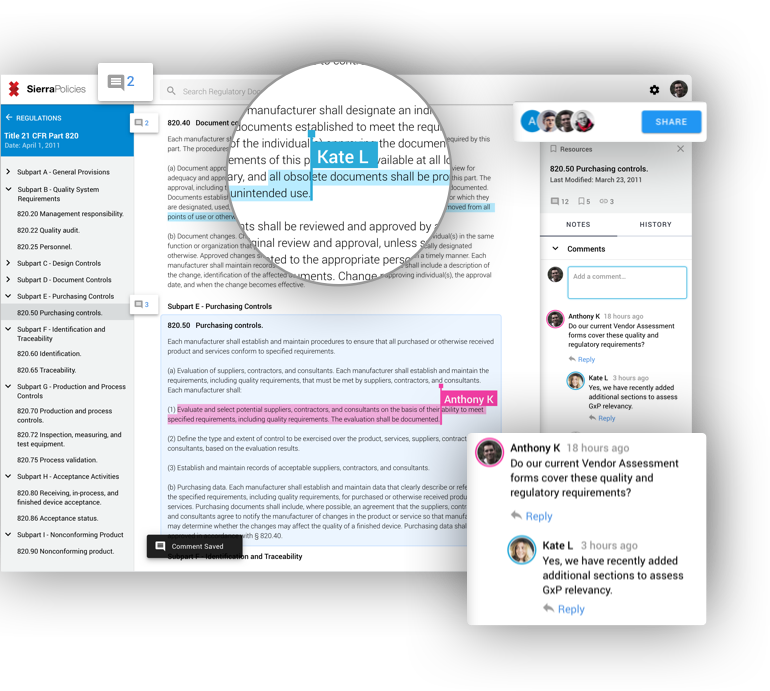
References panel provides links to other regulations and guidelines that are related to the content you’re viewing. The policies database includes cross-references between FDA guidelines and CFRs along with other regulatory standards.
Exports make it easy to email, print, and upload to your Quality Management System. An easy way to prepare policies for presentation in your next audit.

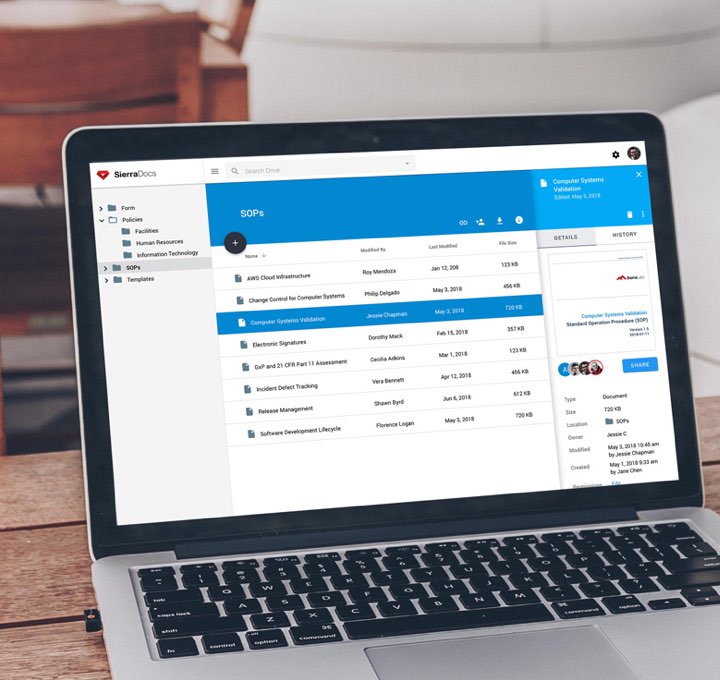
If you are starting from a blank slate, you can save weeks of time by starting with our templates. Already have existing policies and procedures? No problem, upload them and you’re ready to go.
Get a Demo
Turn your policies and procedures into actionable intelligence by integrating with your existing tools to make sure your team is always compliant.
Our system can adapt to any custom workflow and process.
No problem, reach out to us and we can work with you on a custom integration.