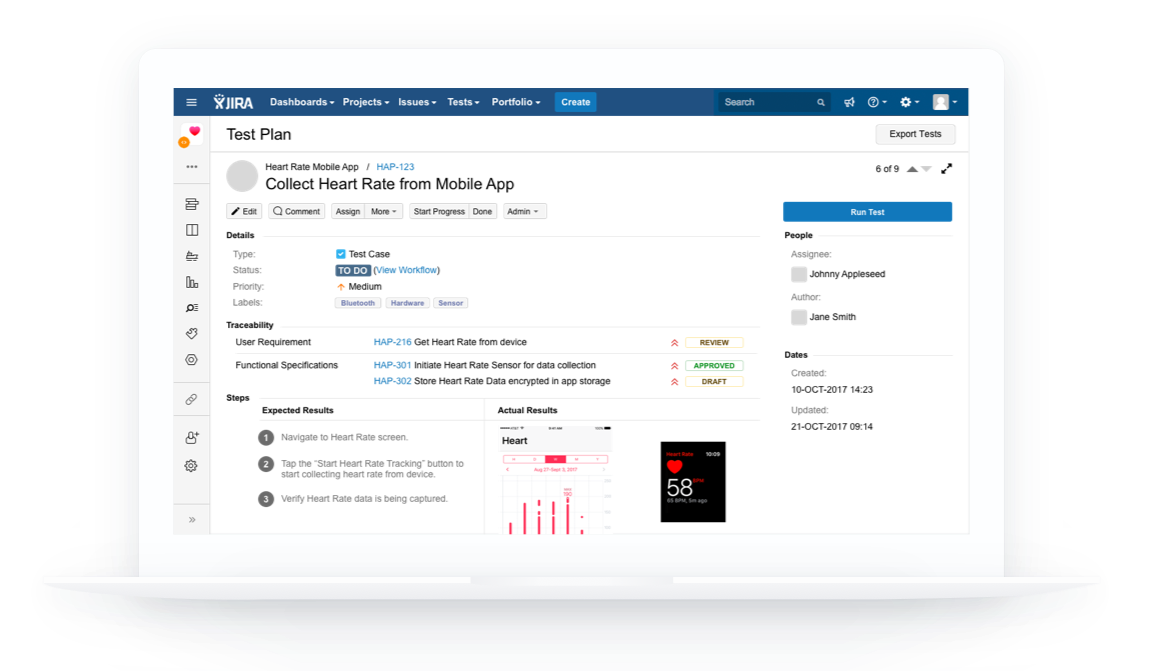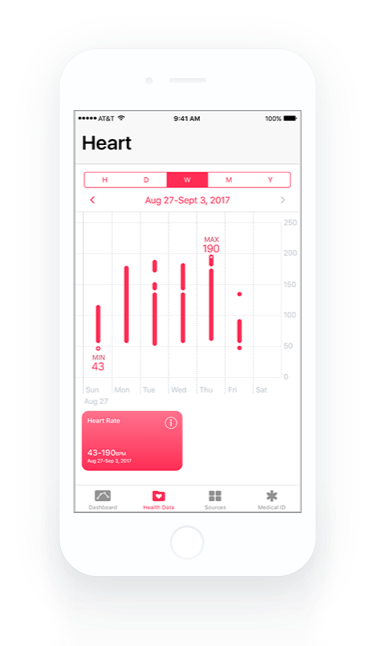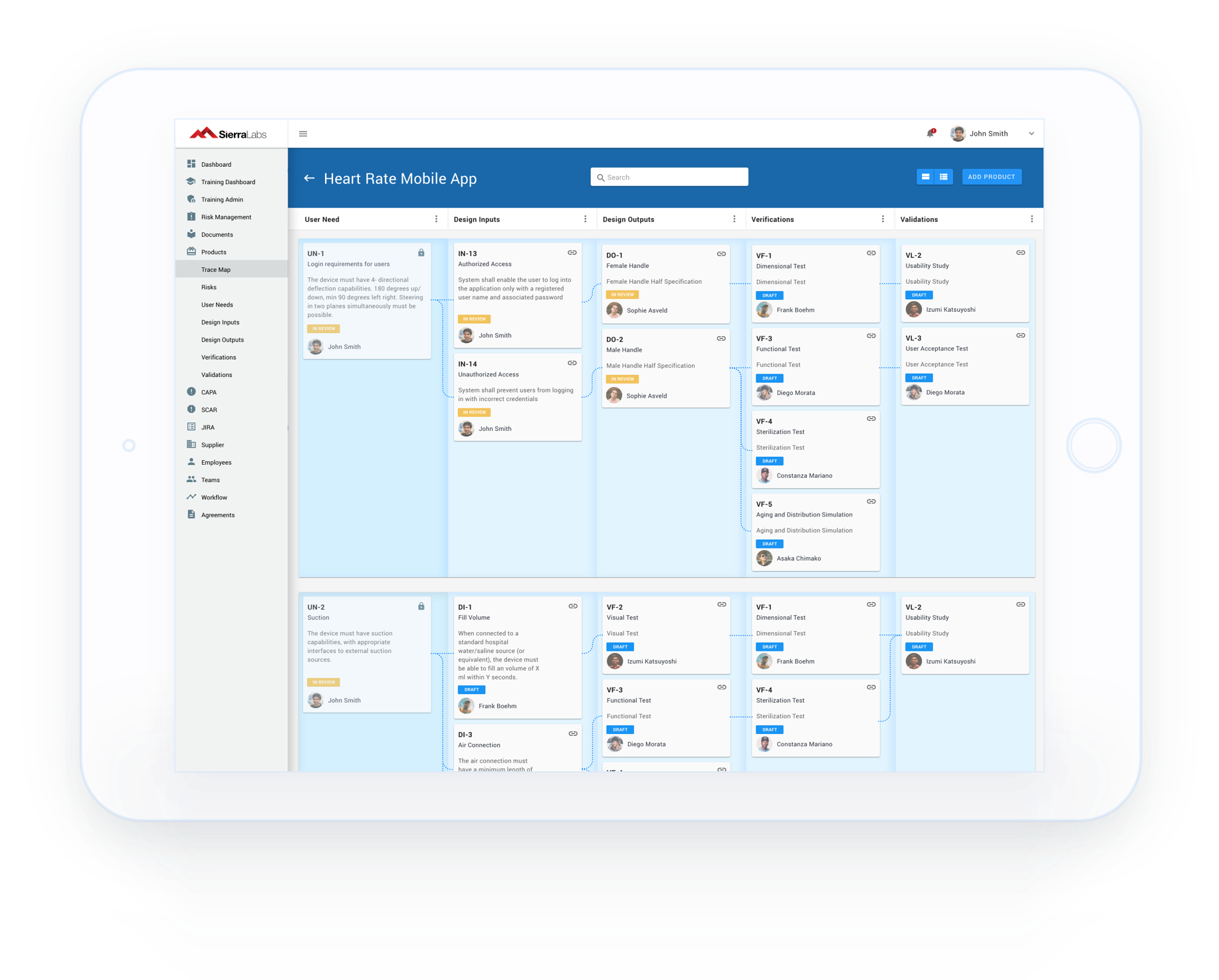
Healthcare and life science Quality Management System that integrates with Atlassian Jira for medical device, biotech, pharmaceutical, and clinical research companies
Download Whitepaper
Designed for healthcare and life science companies of all shapes and sizes.
Workflows designed to help you create everything needed for 510(k) submission and create quality records needed for 21 CFR Part 820 once you're ready to start selling your device.
Track SOPs, GxP system inventories, and all components of your System/Device Life Cycle with workflows designed to comply with 21 CFR Part 11, GLP, GCP, and other industry standards.
Built in tools to draft policies, procedures, and work instructions. Ability to track non-conformance, deviations, and CAPAs with customized quality management reports.
A Quality Management System tailored to fit you that can be deployed in any environment (local servers, cloud, and data centers).
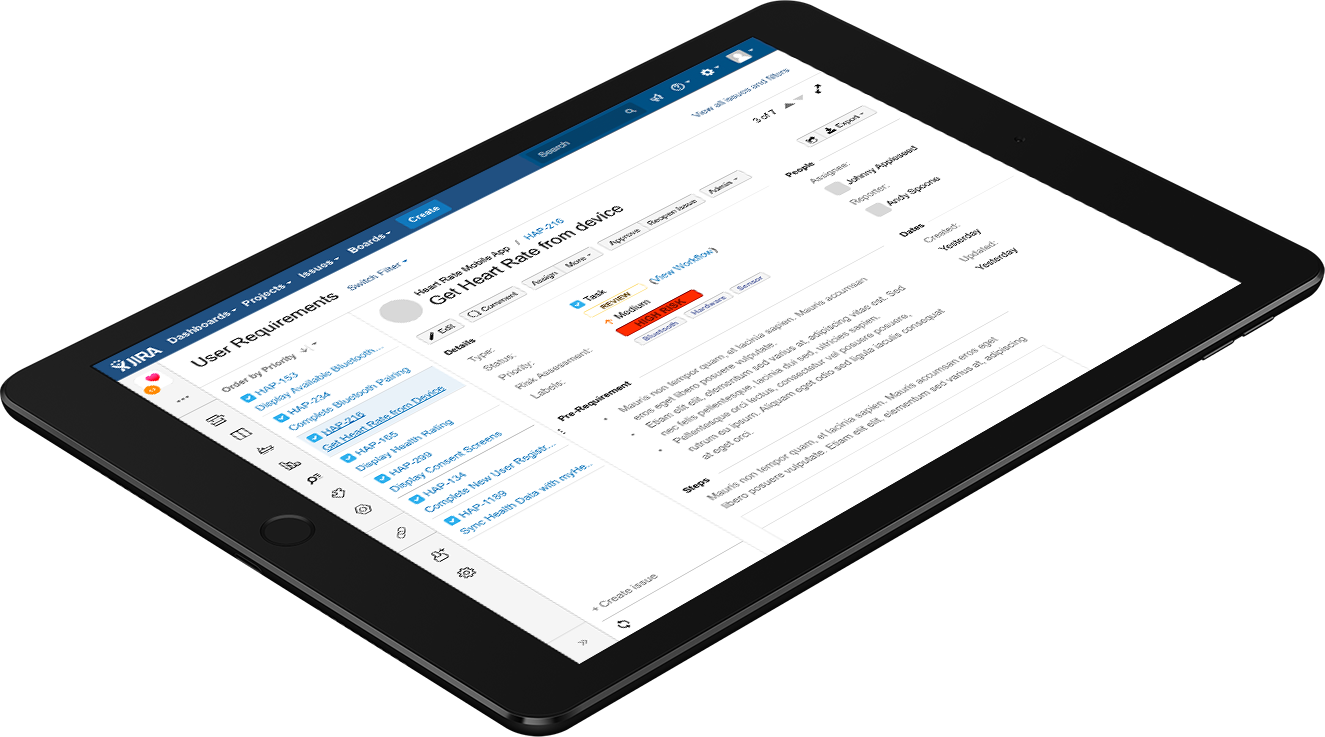
Built to automatically scale with your organization through built in recommendations and workflows
Designed to easily align with current FDA guidance

Generate all your policies, procedures, and artifacts into traditional documents for audit review.
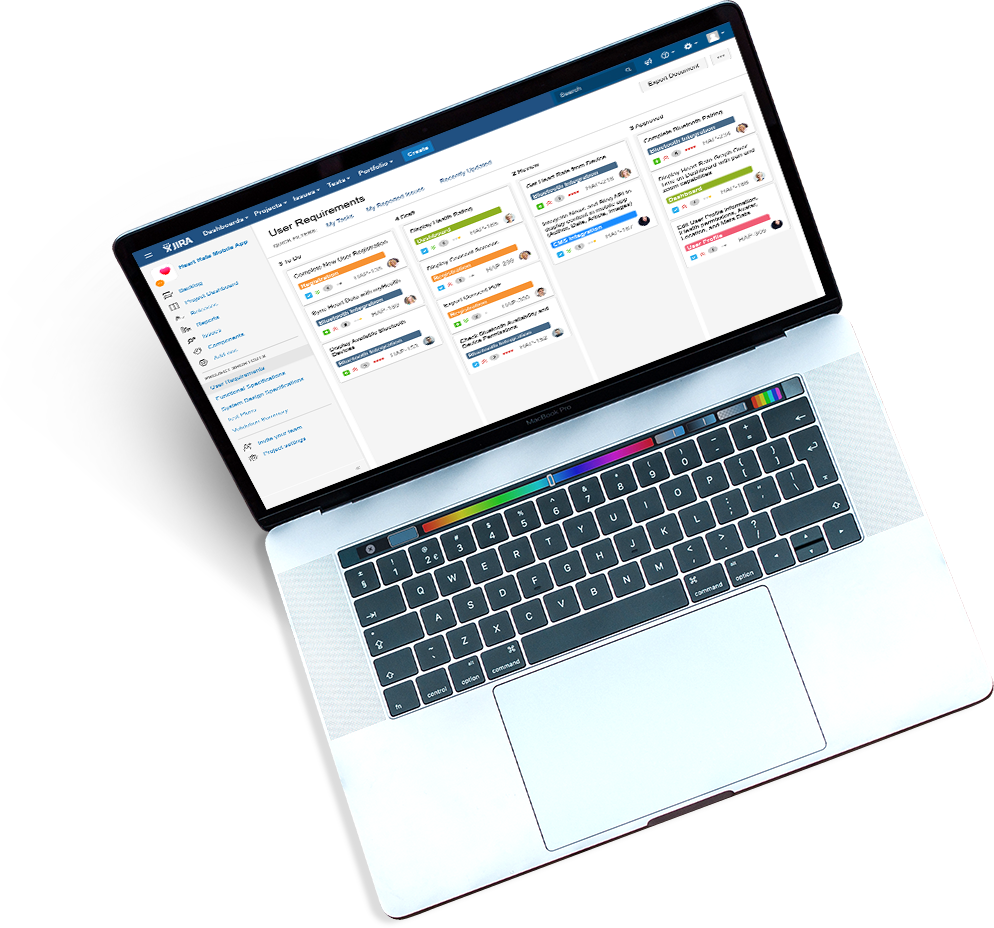


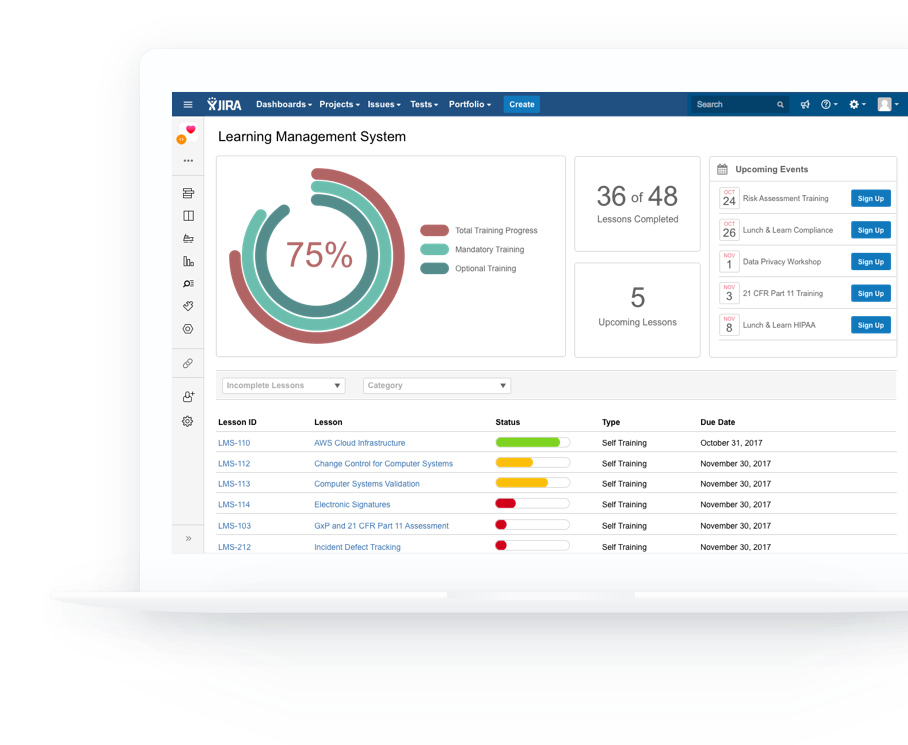
As you scale and improve your workflows, policies and procedures, keep your team trained automatically.

Automate validation testing on devices, apps, web, and custom off the shelf software for your enterprise. Reduce time and resources needed for lengthy testing, reporting, and approvals.